Aqueous liquids
Structural study of water - in ionic liquid microemulsions by SANS
measured by the SANS
Common surfactants form micelles in ionic liquids based on imidazolium cations. Besides, ionic liquid - in - oil microemulsions have been reported. Consisting of bmimBF4, cyclohexane, and the non-ionic surfactant TX100. Earlier SANS studies of such microemulsions proved the formation of surfactant-stabilized dispersed nanodroplets with IL cores.
Water containing microemulsions are much more popular due to practical interest, however, no such systems with ionic liquids have been reported so far. Since ionic liquids show properties similar to oils or organic solvents, one could then expect the formation of water - in - ionic liquid type microemulsions. 1-hexyl-3-methylimidazolium perchlorate (hmimClO4) is an IL immiscible in water. Addition of ionic or non-ionic surfactant in this IL allows the incorporation of water to the mixtures until approximately 10% in weight of water. We have determined the partial phase diagram for the microemulsions using several surfactants. The structure of these microemulsions is however completely unknown to date and we aimed to study it by small-angle neutron scattering. SANS experiments have been carried out on a series of mixtures of IL / surfactant / D2O, varying the water content, and the amount and the type of the surfactant.
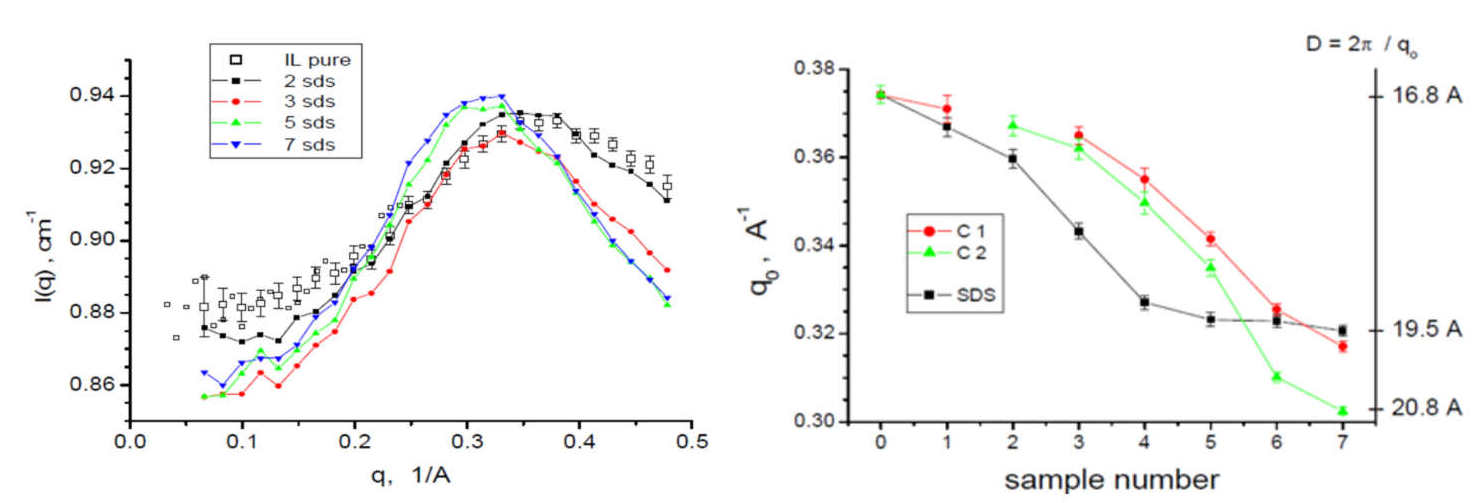
All scattering curves show a broad interference peak, which is qualitatively similar to the structure factor of conventional micro-emulsions. In the same time, it is similar to the structure factor of the neat ionic liquid.
Increasing the water content, the peak position shifts to smaller q, indicating the structural change in the emulsion, this is similar to the behaviour of the conventional water-in-oil emulsions. Therefore, it can be assumed that the present system has a similar inner structure.
