Biological systems
Photosynthetic Membranes
measurements performed on SANS
Thylakoid membranes are the most abundant membranes on earth and possess a central role in photosynthesis by giving place to its light reactions. Our group investigates structural parameters of the thylakoid membranes, isolated from higher plants, in different algal and cyanobacterial cells and in whole leaves with small-angle neutron scattering.
On isolated plant thylakoid membranes we could identify a peak originating from domains of ordered, unappressed stroma lamellae (a part of the highly organised thylakoid membrane assembly), providing information about its averaged repeat distance (RD) values. We found this RD to depend on e.g. the osmolarity and the ionic strength of the suspension medium. We also determined characteristic RDs of thylakoid membranes in algal or cyanobacterial cells and correlated these RDs with the size and the arrangement of the different protein complexes in the thylakoid membranes. Time-resolved SANS measurements were performed on these samples and revealed light-induced reversible reorganizations in the seconds-to-minutes time scale, which appeared to be associated with functional changes in vivo.
The observed changes in the membrane repeat distances are small, especially in living cyanobacterial and algal cells, at most 2 nm, and thus their detection, especially under physiologically relevant conditions, requires use of a non-invasive structure-investigation technique. Our results demonstrate that SANS, which offers good accuracy combined with statistical averaging for the entire, inherently heterogeneous populations of cells and membranes is suitable for this purpose. Recently we could apply this technique for investigating the periodic arrangement of photosynthetic membranes in intact leaves.
We have also investigated photosystem II membrane fragments by elastic incoherent neutron scattering, revealing a hydration-dependent transition which is correlated with the detachment of the oxygen evolving complex from the membrane.
Our group works in strong collaboration with the Laboratory of Photosynthetic Membranes at the Biological Research Center (Szeged, Hungary), where sample characterization with complementary biophysical tools is also performed, and biologically relevant information is obtained from the experiments by relying on the group’s plant science expertise.
To extend the parameter range of our BNC instruments part of the above experiments we performed at large scale facilities, such as the Institut Laue-Langevin (France) and the Paul Scherrer Institute (Switzerland).
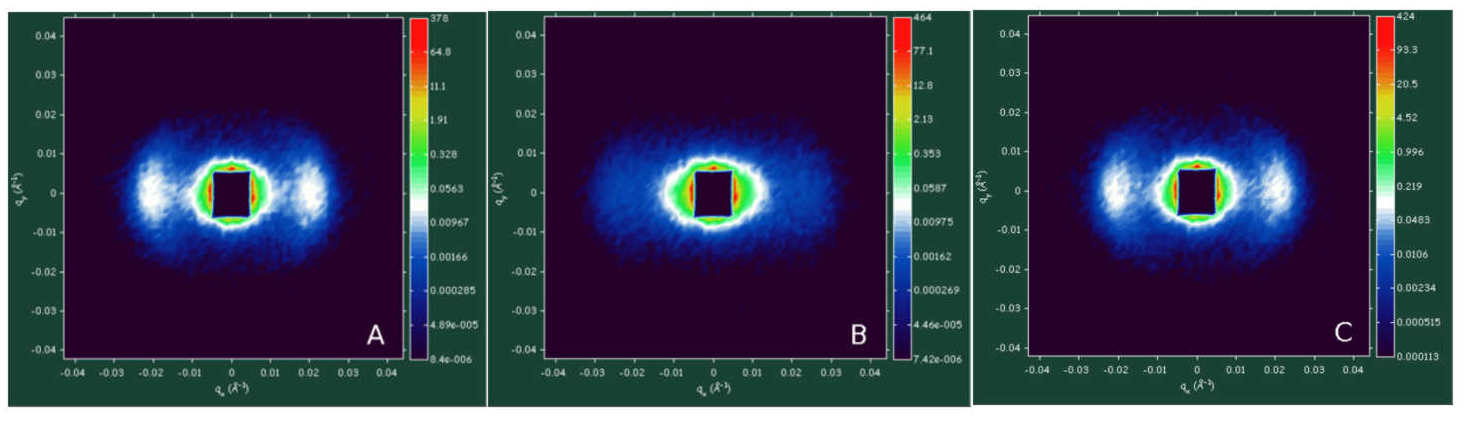
Figure 1. Effect of illumination on the two-dimensional SANS profile of magnetically oriented spinach thylakoid membranes, recorded with the 2D detector of the D22 SANS instrument (ILL). Dark-adapted state (A), after illumination with white light of 1700 μmol photons m-2 s-1 photon flux density for 5 min (B), after 5 min light and 5 min dark (C).
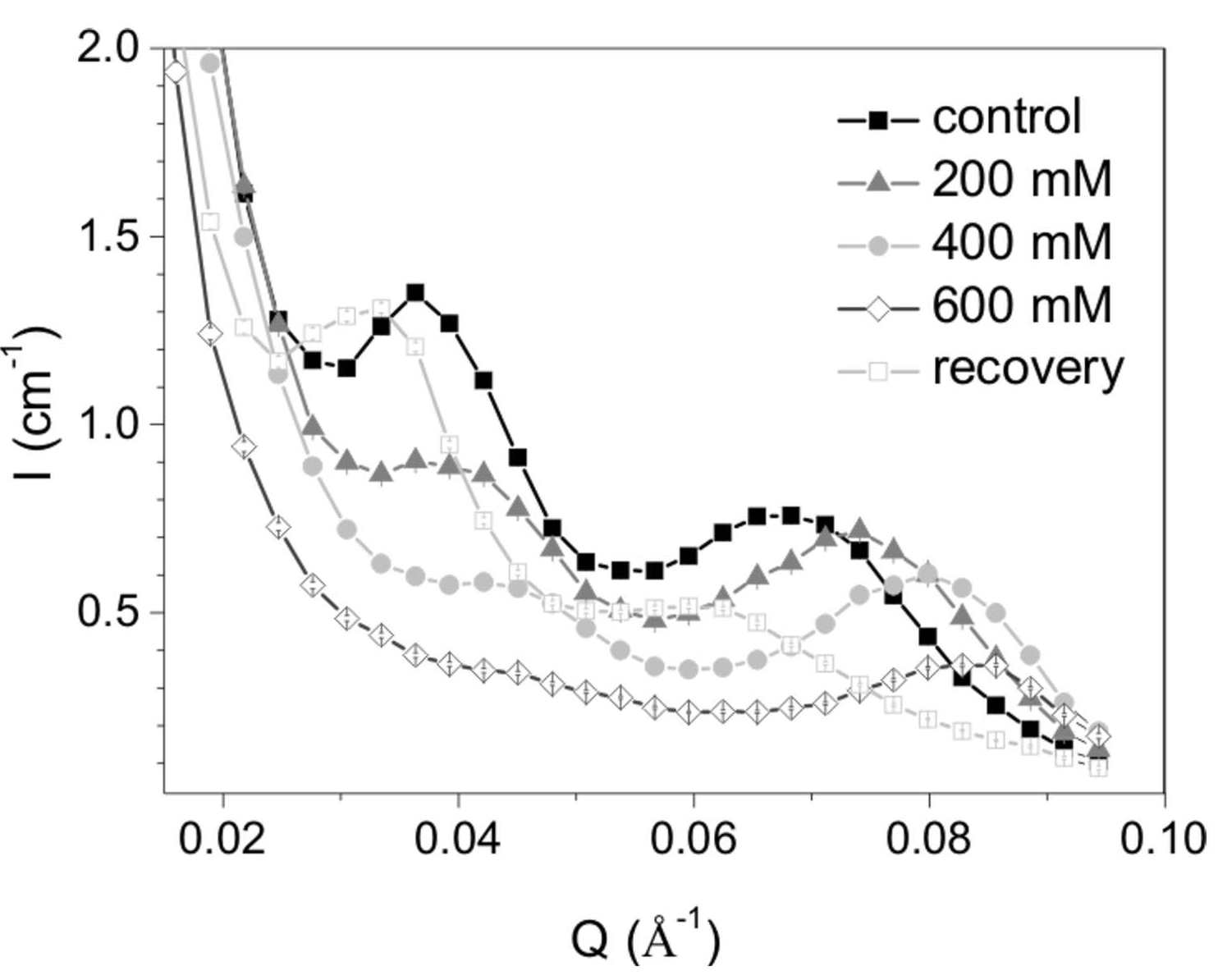
Figure 2. Effect of osmolarity on algal (Phaeodactylum tricornutum) cells; SANS profiles recorded on the "Yellow Submarine" SANS instrument (BNC).
Recommended articles:
Várkonyi Zs., Nagy G., Lambrev P., Kiss A.Z., Székely N.K., Rosta L., Garab Gy., Effect of phosphorylation on the thermal and light stability of the thylakoid membranes Photosynth Res 99 (2009) 161-171
Nagy, G., Posselt, D., Kovács, L., Holm, J. K., Szabó, M., Ughy, B., Rosta, L., Peters, J., Timmins, P. and Garab, G. (2011) Reversible membrane reorganizations during photosynthesis in vivo: revealed by small-angle neutron scattering. Biochem. J. (Accelerated Publication) 436: 225-23
Posselt, D., Nagy, G., Kirkensgaard, J. J. K., Holm, J. K., Aagaard, T. H., Timmins, P., Rétfalvi, E., Rosta, L., Kovács, L. and Garab, G. (2012) Small-angle neutron scattering study of the ultrastructure of chloroplast thylakoid membranes – periodicity and structural flexibility of the stroma lamellae Biochim. Biophys. Acta. – Bioenerg. 1817:1220-1228
Nagy, G., Szabó, M., Ünnep, R., Káli, G., Miloslavina, Y., Lambrev, P. H., Zsiros, O., Porcar, L., Rosta, L. and Garab, G. (2012) Modulation of the multilamellar membrane organization and of the chiral macrodomains in the diatom Phaeodactylum tricornutum revealed by small-angle neutron scattering and circular dichroism spectroscopy. Photosynth. Res. 111:71-79
Nagy, G., Kovács, L., Ünnep, R., Zsiros, O., Almásy, L., Rosta, L., Timmins, P., Peters, J., Posselt, D. and Garab, G. (2013) Kinetics of structural reorganizations in multilamellar photosynthetic membranes monitored by small angle neutron scattering. Eur. Phys. J. E Soft Matter 36:69
Nagy, G., Ünnep, R., Zsiros, O., Tokutsu, R., Takizawa, K., Porcar, L., Moyet, L., Petroutsos, D., Garab, G., Finazzi, G. and Minagawa, J. (2014) Chloroplast remodeling during state transitions in Chlamydomonas reinhardtii as revealed by noninvasive techniques in vivo. Proc. Natl. Acad. Sci. U.S.A. 111:5042-5047
Ünnep, R., Zsiros, O., Solymosi, K., Kovács, L, Lambrev, P.H., Tóth, T., Schweins, R., Posselt, D., Székely, N.K., Rosta, L., Nagy, G. and Garab, G. (2014) The ultrastructure and flexibility of thylakoid membranes in leaves and isolated chloroplasts as revealed by small-angle neutron scattering. Biochim. Biophys. Acta. – Bioenerg. S0005-2728:00027-9
Ünnep, R., Nagy, G., Markó, M. and Garab, G. (2014) Monitoring thylakoid ultrastructural changes in vivo using small-angle neutron scattering. Plant. Physiol. Biochem. S0981-9428:00049-7
G. Nagy, G. Garab, J. Pieper, Neutron Scattering in Photosynthesis Research, in Contemporary Problems of Photosynthesis, Moscow (Eds: S. I. Allakhverdiev, A. B. Rubin and V. A. Shuvalov) Izhevsk Institute of Computer Science (2014)
