Disordered materials
Crystalline vs. Amorphous
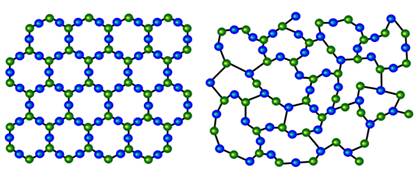
Crystalline solids exhibit translational symmetry (periodicity of atomic positions, unit cell), while in
amorphous (glassy, or non-crystalline solids) lack the long-range order, but a short-range order still exists.
Types of disordered solid materials
Amorphous solids
Certain thin films: semiconductors, metals...
Glasses (supercooled liquids): Vitrious silica, oxide glasses, chalcogenide glasses
Composites: random alloys, metal-insulator mixtures, porous materials
Amorphous solids at intermediate length scales often exhibit fractal structure source: G. A. Niklasson
